CSR Initiatives to Strengthen Peer Review
CSR is committed to strengthening the peer review process. Learn about our commitment and relevant data.
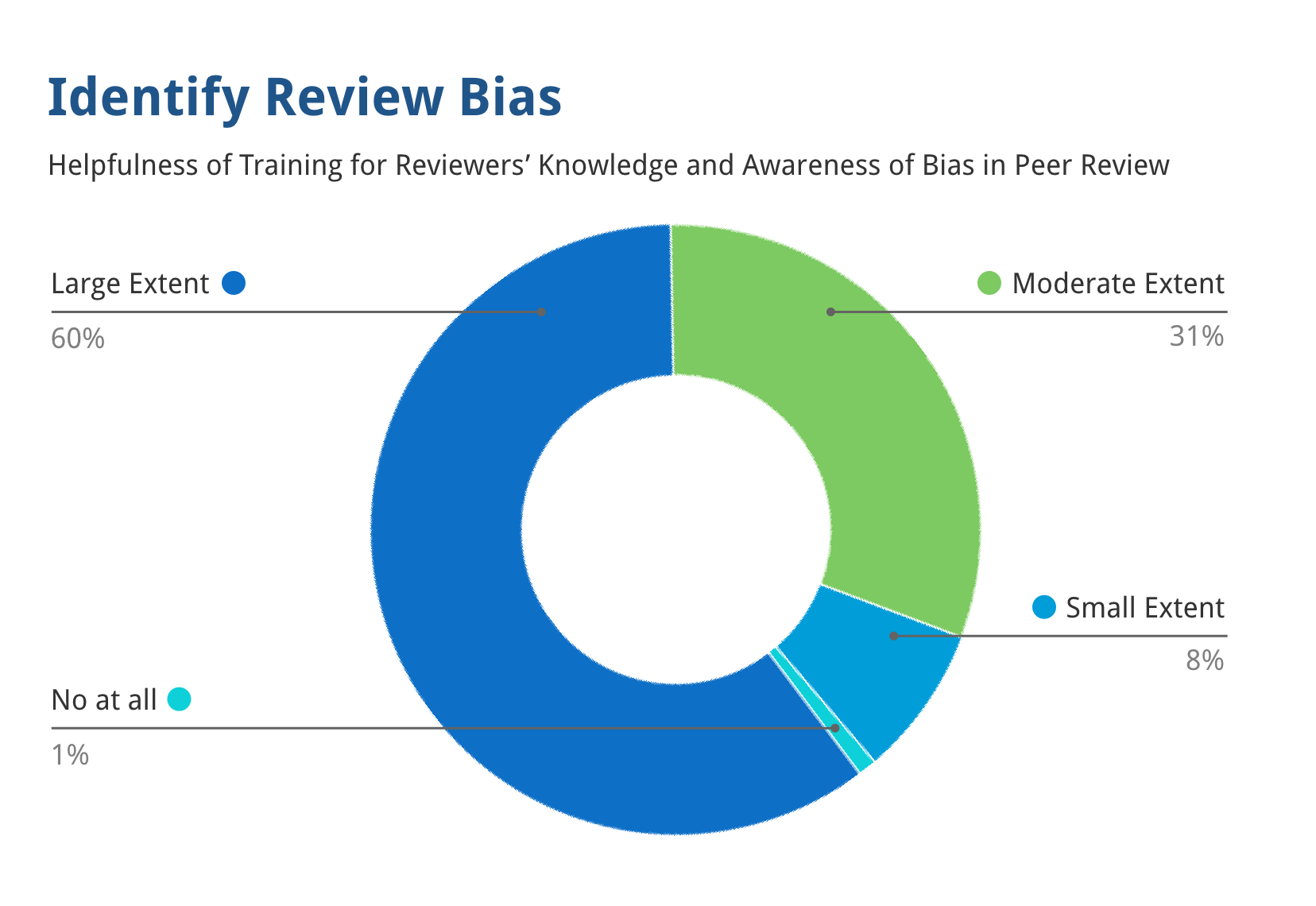
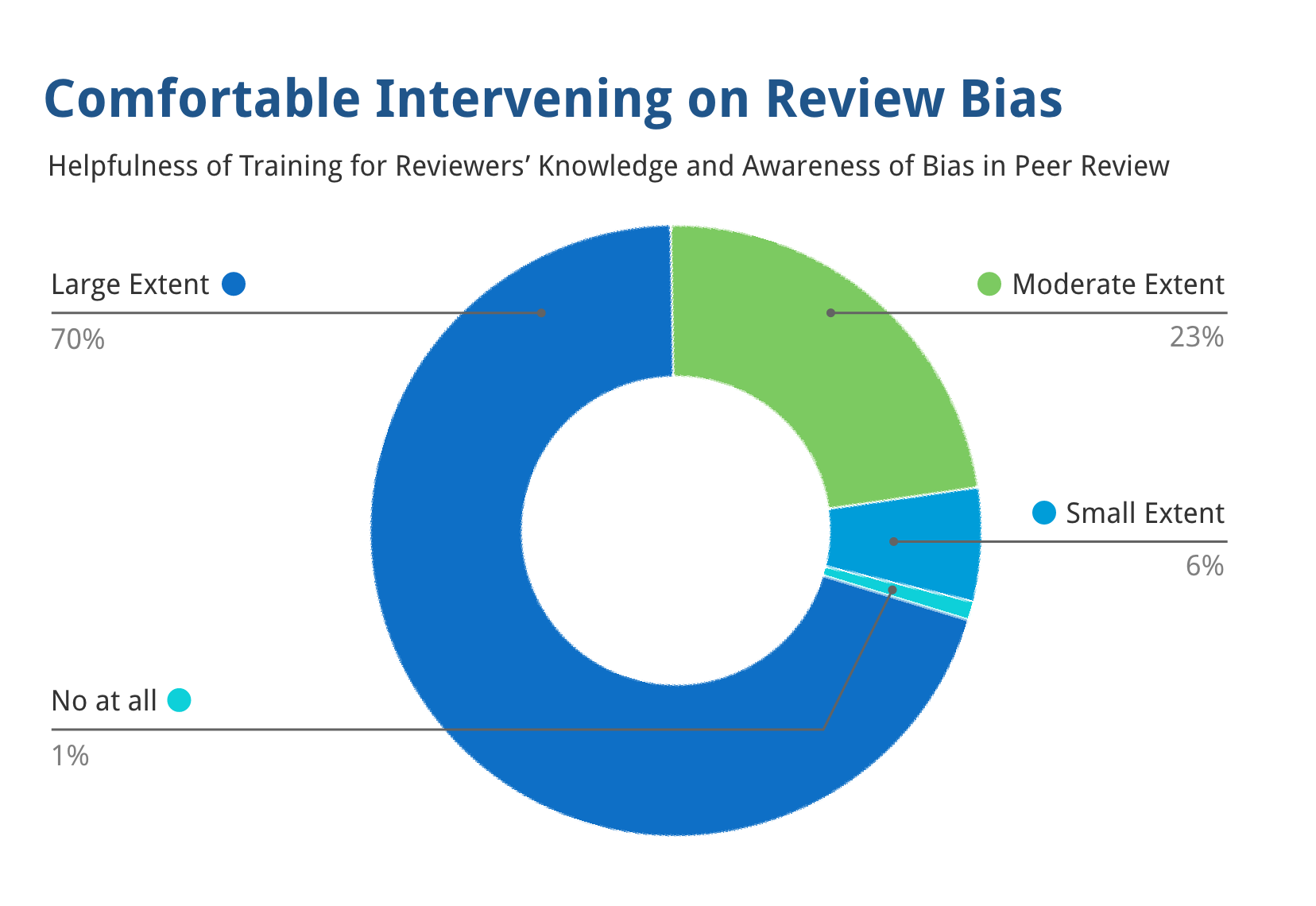
Training to Mitigate Unfairness in Peer Review for Reviewers, Chairs, and SROs
>45,000
As of July 2025, more than 45,000 reviewers have completed the training.
91%
91% of reviewers thought that the training substantially improved their ability to identify bias in peer review.
93%
93% of reviewers stated the training made them substantially more comfortable intervening against bias.
CSR developed training specifically targeted toward mitigating the most common biases in the peer review process. The training includes personal testimonials, interactive exercises, and a narrated mock study section demonstrating techniques to intervene – all based on real-life examples. The training was developed with the assistance of the CSR Advisory Council Working Group. Training has been provided to all CSR reviewers since August 2021.
Return to top
Reporting Avenues for Unfair Reviews, Uncivil Conduct on Panels
CSR launched a widely-publicized reporting avenue for issues related to respectful interactions or anything else that could affect the fairness of the review process. The reporting channel is open to all – investigators, reviewers, and program staff.
Investigate
Every allegation is carefully investigated by CSR senior management.
Resolve
If we agree the review was flawed, CSR will re-review application in the same council round. If we don’t agree, the official NIH appeals process remains available to all investigators.
Closure and Culture Change
Re-review? CSR Scientific Division Director discusses the issue with the reviewer, if appropriate. Actions might also include not inviting the reviewer to serve in the future.

Report a Concern
Send a message to
reportconcern@csr.nih.gov
Return to top
Exploring Changes to Review to Make it More Fair and Effective

Leading efforts to simplify review criteria
CSR led efforts to simplify the NIH peer review criteria framework for the majority of research project grants. The aim is to help focus reviewers on the key questions needed to assess the scientific and technical merit of the research proposals: Should and can the proposed research be conducted? NIH’s ultimate goal through this initiative is to identify the best, most innovative science with the potential to improve human health or advance our scientific understanding. In forming the simplified framework, NIH gathered input through a Request for Information, receiving more than 800 responses from individuals, scientific societies, and academic institutions. The recommendations were developed with guidance from the CSR Advisory Council (through the Clinical Trials, Non-Clinical Trials working groups). The new framework became effective for all applications with due dates on or after January 25, 2025, and beginning with the summer 2025 review meetings.

Improving Fellowship Review
CSR led efforts to improve the fellowship application and review process. The goal is to help reviewers better evaluate the trainee’s potential and the quality of the scientific training plan without the undue influence of the reputation of either the sponsor or the institution. Through this effort, NIH aims to improve the chances that the most promising fellowship candidates will be consistently identified by scientific review panels. The modified review criteria, developed with guidance from the CSR Advisory Council Working Group on Fellowship Review and from community input through a Request for Information, were established to better focus reviewers on three key assessments: (1) the potential and preparedness of the applicant; (2) commitment to the candidate; (3) the quality of the training plan. Coursework grades will no longer be considered. Parent Notices of Funding Opportunity with the modified review criteria for fellowships have been published. Applications submitted on or after August 8 will have to use the new forms, and fall 2025 review meetings will follow the new criteria.






